Introduction
In the evolving landscape of healthcare and clinical research, access to timely and accurate data is essential. Researchers, pharmaceutical companies, and healthcare organizations require reliable information to optimize trials, monitor competitor studies, and assess patient outcomes. The Clinical Trials Data Scraper API enables the collection of structured, real-time trial data from multiple registries efficiently. By leveraging web scraping API technology, organizations can automate the extraction of trial metadata, study designs, results, and publications, eliminating the need for manual data gathering.
With features like real-time clinical trials data extraction and integration capabilities for analytics platforms, this API supports comprehensive market research and enables strategic decision-making. It allows teams to monitor ongoing and completed trials, extract relevant metrics, and identify trends across therapeutic areas. Combining the power of clinical study API scraping service and clinical trial registry scraping tool, the API helps researchers maintain a competitive edge while improving accuracy and efficiency.
Additionally, the Clinical Trials Data Scraper API supports research optimization via trial data APIs, offering actionable insights that enhance productivity and accelerate clinical decision-making.
Growth of Clinical Trial Registrations
Between 2020 and 2025, global clinical trial registrations have grown steadily, reflecting increased research activity across therapeutic areas. Using the Clinical Trials Data Scraper API, researchers can scrape clinical research API data to track this growth in real time.
Table 1: Global Clinical Trial Registrations (2020–2025)
| Year | Trials Registered | Avg Duration (Months) | Countries Covered |
|---|---|---|---|
| 2020 | 42,000 | 24 | 85 |
| 2021 | 45,500 | 23 | 90 |
| 2022 | 50,000 | 22 | 95 |
| 2023 | 55,200 | 21 | 100 |
| 2024 | 60,000 | 20 | 105 |
| 2025 | 65,500 | 19 | 110 |
The API enables the extraction of trial counts, duration trends, and geographic distribution efficiently, allowing data-driven research planning and extract clinical trial results for research purposes.
Therapeutic Area Insights
Healthcare researchers benefit from detailed insights by therapeutic area. The Clinical Trials Data Scraper API supports clinical study API scraping service capabilities to categorize trials by indications such as oncology, cardiology, and neurology.
Table 2: Clinical Trials by Therapeutic Area (2020–2025)
| Year | Oncology | Cardiology | Neurology | Others |
|---|---|---|---|---|
| 2020 | 10,000 | 8,000 | 5,000 | 19,000 |
| 2021 | 11,200 | 8,500 | 5,400 | 20,400 |
| 2022 | 12,500 | 9,000 | 5,900 | 22,600 |
| 2023 | 14,000 | 9,500 | 6,300 | 25,400 |
| 2024 | 15,500 | 10,000 | 6,800 | 27,700 |
| 2025 | 17,000 | 10,500 | 7,200 | 30,800 |
By leveraging real-time clinical trials data extraction, research teams can detect emerging trends and focus on high-growth therapeutic areas.
Unlock therapeutic area insights with our Clinical Trials Data Scraper API to track trends, monitor studies, and optimize research efficiently.
Get Insights Now!Trial Phases Analysis
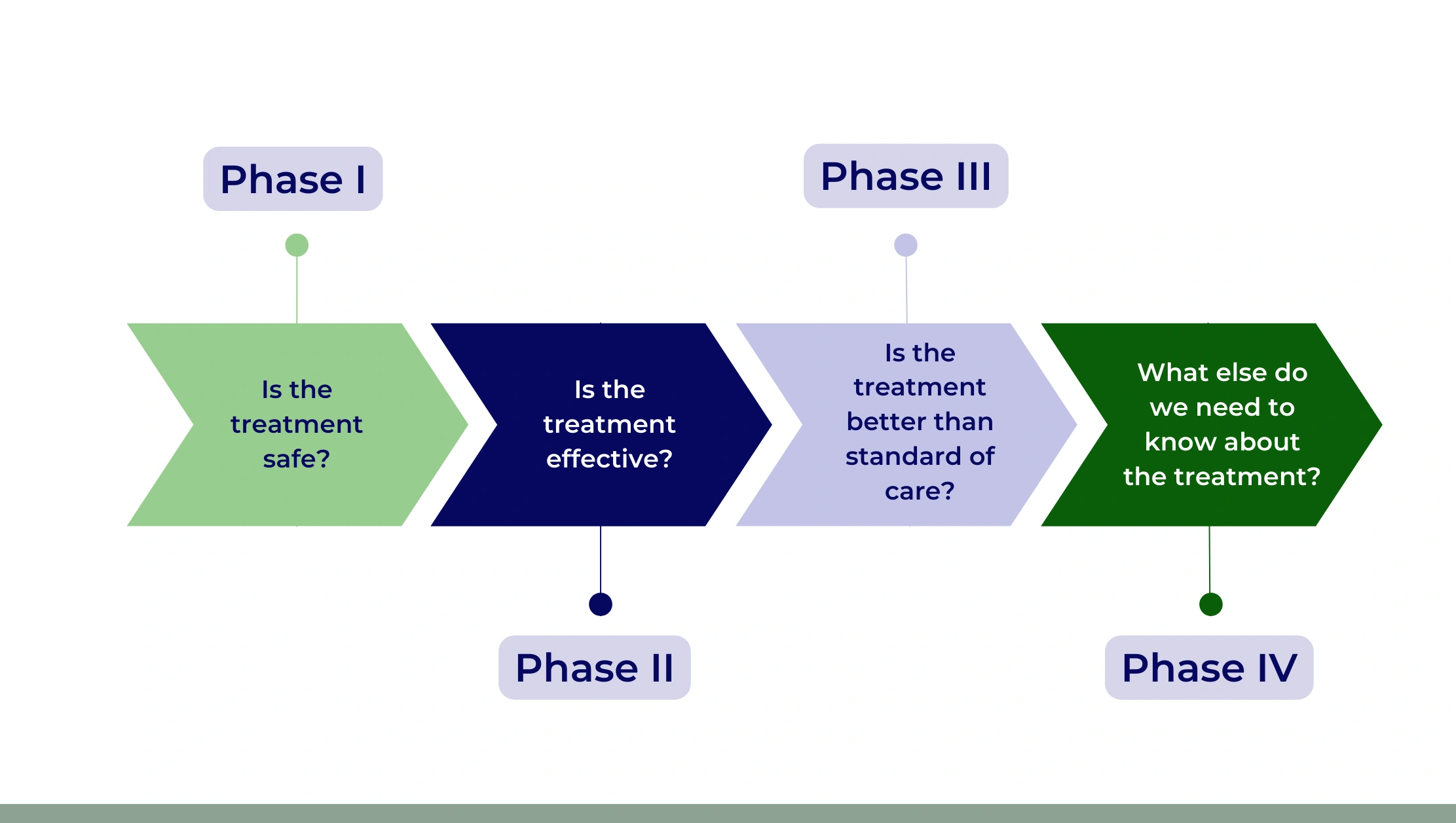
The API enables monitoring of trial phases (Phase I-IV) and their distribution over time. Automated extraction allows for clinical trial registry scraping tool utilization to analyze phase durations, success rates, and participant enrollment.
Table 3: Trials by Phase (2020–2025)
| Year | Phase I | Phase II | Phase III | Phase IV |
|---|---|---|---|---|
| 2020 | 12,000 | 15,000 | 10,000 | 5,000 |
| 2021 | 12,500 | 16,000 | 11,000 | 6,000 |
| 2022 | 13,500 | 17,000 | 12,000 | 7,000 |
| 2023 | 14,500 | 18,500 | 13,000 | 9,000 |
| 2024 | 16,000 | 20,000 | 14,000 | 10,000 |
| 2025 | 17,500 | 21,500 | 15,500 | 11,000 |
Results and Outcomes Tracking
Using extract clinical trial results for research, teams can monitor efficacy endpoints, adverse events, and success rates. This ensures evidence-based decision-making and improved trial design.
Table 4: Trial Success Rates (2020–2025)
| Year | Success Rate (%) | Avg Participants | Avg Duration (Months) |
|---|---|---|---|
| 2020 | 65 | 250 | 24 |
| 2021 | 66 | 260 | 23 |
| 2022 | 67 | 270 | 22 |
| 2023 | 68 | 280 | 21 |
| 2024 | 69 | 290 | 20 |
| 2025 | 70 | 300 | 19 |
Global vs Regional Trends

The API allows comparisons between global and regional trials. By leveraging clinical study API scraping service, organizations can monitor country-specific growth and international collaboration.
Table 5: Trials by Region (2020–2025)
| Year | North America | Europe | Asia | Others |
|---|---|---|---|---|
| 2020 | 15,000 | 12,000 | 10,000 | 5,000 |
| 2021 | 16,000 | 12,500 | 11,000 | 6,000 |
| 2022 | 17,500 | 13,000 | 12,500 | 7,000 |
| 2023 | 19,000 | 14,000 | 14,000 | 8,000 |
| 2024 | 20,500 | 15,000 | 15,500 | 9,000 |
| 2025 | 22,000 | 16,000 | 17,000 | 10,500 |
Compare global and regional clinical trial trends using our Clinical Trials Data Scraper API to make informed, data-driven research decisions.—
Get Insights Now!Integration and Automation Benefits

Automated Healthcare Data Scraping API integration reduces manual effort and increases data reliability. Researchers can scrape clinical research API data and integrate with analytics platforms for continuous monitoring and trend analysis.
Why Choose Real Data API?
Real Data API provides comprehensive access to clinical trial datasets with precision and speed. By leveraging the Clinical Trials Data Scraper API, researchers can perform real-time clinical trials data extraction efficiently. The platform enables research optimization via trial data APIs, ensuring teams focus on analysis rather than manual collection. Its clinical trial registry scraping tool and clinical study API scraping service features allow structured extraction of trial results, participant demographics, endpoints, and adverse events. Integration with Healthcare Data Scraping API ensures seamless workflow management, facilitating market research and competitive analysis.
With Real Data API, organizations can consolidate trial information from multiple sources, access historical and ongoing data, and generate insights for strategic decision-making. Its automation, accuracy, and scalability make it ideal for researchers, pharmaceutical companies, and healthcare analysts seeking actionable intelligence.
Conclusion
The Clinical Trials Data Scraper API empowers healthcare organizations to access, analyze, and act on clinical trial data efficiently. By using this API, teams can scrape clinical research API data, implement clinical trial registry scraping tool features, and extract clinical trial results for research in real-time. Automated workflows reduce errors, enhance productivity, and support research optimization via trial data APIs.
Researchers, pharmaceutical companies, and analysts can monitor trends, evaluate competitor trials, and gain actionable insights for publication, study design, and strategic planning. Integrating Healthcare Data Scraping API and leveraging structured, up-to-date datasets ensures reliable and accurate decision-making.
Unlock the full potential of clinical research data with Real Data API today. Automate your scraping of clinical trials API data, gain real-time insights, and accelerate research efficiency for better healthcare outcomes.